The complement system found in the blood of mammals is composed of heat labile substances (proteins) that combine with antibodies or cell surfaces. This complex, multicomponent system is composed of about 26 proteins. The "complement cascade" is constitutive and non-specific but it must be activated in order to function. The functions of complement include:
Most of the complement components are numbered (e.g. C1, C2, C3, etc.) but some are simply refered to as "Factors". Some of the components must be enzymatically cleaved to activate their function; others simply combine to form complexes that are active. The following table lists these components and their functions.
| Components of the Classical Pathway | ||
|---|---|---|
| Native component | Active component(s) | Function(s) |
| C1(q,r,s) | C1q | Binds to antibody that has bound antigen, activates C1r. |
| C1r | Cleaves C1s to activate protease function. | |
| C1s | Cleaves C2 and C4. | |
| C2 | C2a | Active enzyme of classical pathway; cleaves C3 and C5. |
| C2b | Unknown. | |
| C3 | C3a | Mediates inflammation; anaphylatoxin. |
| C3b | Binds C5 for cleavage by C2a. Binds cell surfaces for opsonization and activation of alternate pathway. |
|
| C4 | C4a | Mediates inflammation. |
| C4b | Binds C2 for cleavage by C1s. Binds cell surfaces for opsonization. | |
| Components of the Alternate Pathway | ||
| Native component | Active component(s) | Function(s) |
| C3 | C3a | Mediates inflammation; anaphylatoxin. |
| C3b | Binds cell surfaces for opsonization and activation of alternate pathway. | |
| Factor B | B | Binds membrane bound C3b. Cleaved by Factor D. |
| Ba | Unknown. | |
| Bb | Cleaved form stabilized by P produces C3 convertase. | |
| Factor D | D | Cleaves Factor B when bound to C3b. |
| Properdin | P | Binds and stabilizes membrane bound C3bBb. |
| Components of the Membrane-Attack Complex | ||
| Native component | Active component(s) | Function(s) |
| C5 | C5a | Mediates inflammation; anaphylatoxin, chemotaxin. |
| C5b | Initiates assembly of the membrane-attack complex (MAC). | |
| C6 | C6 | Binds C5b, forms acceptor for C7. |
| C7 | C7 | Binds C5b6, inserts into membrane, forms acceptor for C8. |
| C8 | C8 | Binds C5b67, initiates C9 polymerization. |
| C9 | C9n | Polymerizes around C5b678 to form channel that causes cell lysis. |
Classical Pathway
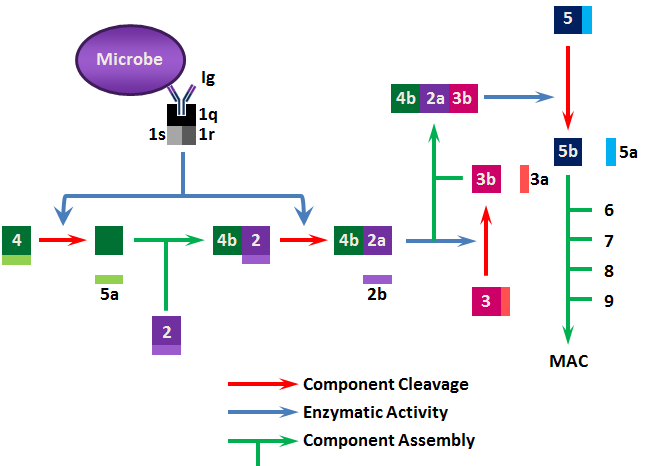
The classical pathway starts with C1; C1 binds to immunoglobulin Fc (primarily IgM and IgG); C1 is recognition complex composed of 22 polypeptide chains in 3 subunits; C1q, C1r, C1s. C1q is the actual recognition portion, a glycoprotein containing hydroxyproline and hydroxylysine that looks like a tulip flower. Upon binding via C1q, C1r is activated to become a protease that cleaves C1s to a form that activates (cleaves) both C2 and C4 to C2a/b and C4a/b. C2a and C4b combine to produce C3 convertase (C3 activating enzyme). C4a has anaphylactic activity (inflammatory response).
C3 is central to both the classical and alternative pathways. In classical, C4b2a convertase cleaves C3 into C3a/b. C3a is a potent anaphylatoxin. C3b combines with C4b2a to form C4b2a3b complex that is a C5 convertase. C3b can also bind directly to cells making them susceptible to phagocytosis.
C5 is converted by C5 convertase (i.e. C4b2a3b) to C5a/b. C5a has potent anaphylatoxic and chemotaxic activities. C5b functions as an anchor on the target cell surface to which the lytic membrane-attack complex (MAC) forms. MAC includes C5b, C6, C7, C8 and C9. Once C9 polymerizes to form a hole in the cell wall, lysis ensues.

Generally, only Gram-negative cells can be directly lysed by antibody plus complement; Gram-positive cells are mostly resistant. However, phagocytosis is greatly enhanced by C3b binding (phagocytes have C3b receptors on their surface) and antibody is not always required. In addition, complement can neutralize virus particles either by direct lysis or by preventing viral penetration of host cells.
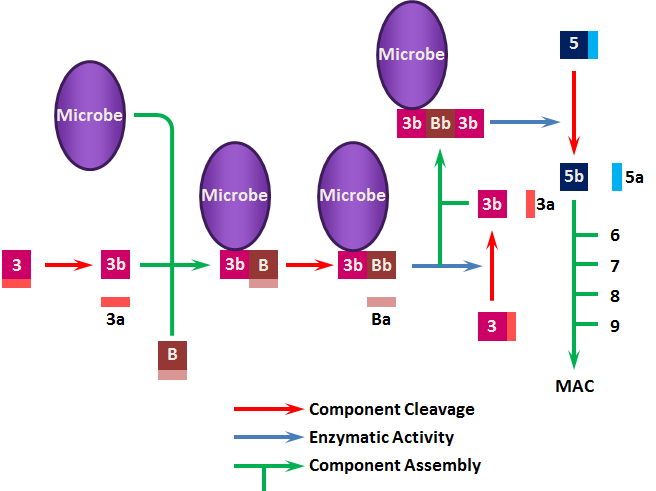
Because both the classical and alternate pathways depend upon C3b, regulation of the complement cascade is mediated via 3 proteins that affect the levels and activities of this component.