Immunoglobulins generally assume one of two roles: immunoglobulins may act as i) plasma membrane bound antigen receptors on the surface of a B-cell or ii) as antibodies free in cellular fluids functioning to intercept and eliminate antigenic determinants. In either role, antibody function is intimately related to its structure and this page will introduce immunoglobulins (antibodies) and relate their structure to their function in host defense.

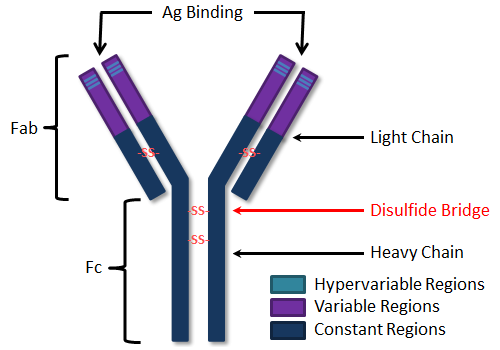
Immunoglobulins are composed of four polypeptide chains: two "light" chains (lambda or kappa), and two "heavy" chains (alpha, delta, gamma, epsilon or mu). The type of heavy chain determines the immunoglobulin isotype (IgA, IgD, IgG, IgE, IgM, respectively). Light chains are composed of 220 amino acid residues while heavy chains are composed of 440-550 amino acids. Each chain has "constant" and "variable" regions as shown in the figure. Variable regions are contained within the amino (NH2) terminal end of the polypeptide chain (amino acids 1-110). When comparing one antibody to another, these amino acid sequences are quite distinct. Constant regions, comprising amino acids 111-220 (or 440-550), are rather uniform, in comparison, from one antibody to another, within the same isotype. "Hypervariable" regions, or "Complementarity Determining Regions" (CDRs) are found within the variable regions of both the heavy and light chains. These regions serve to recognize and bind specifically to antigen. The four polypeptide chains are held together by covalent disulfide (-S-S-) bonds.
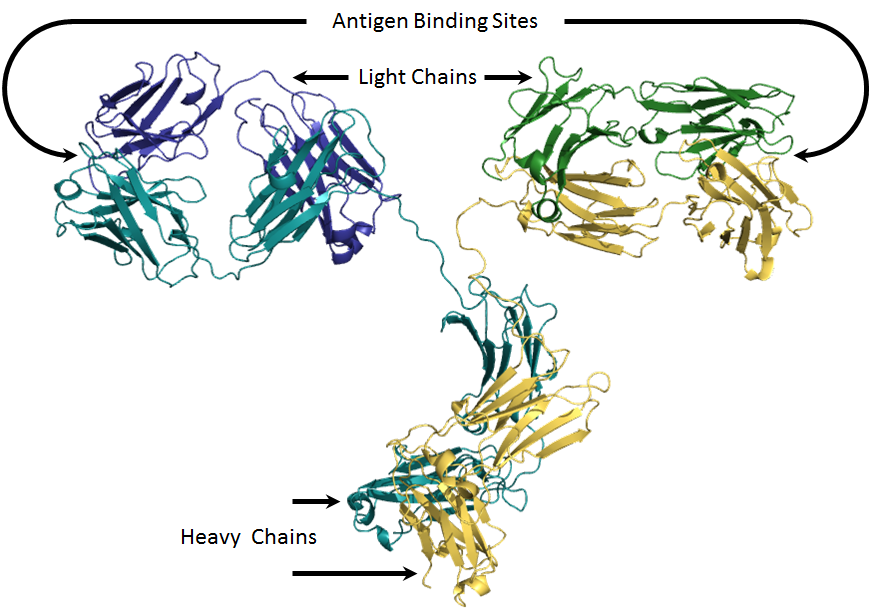
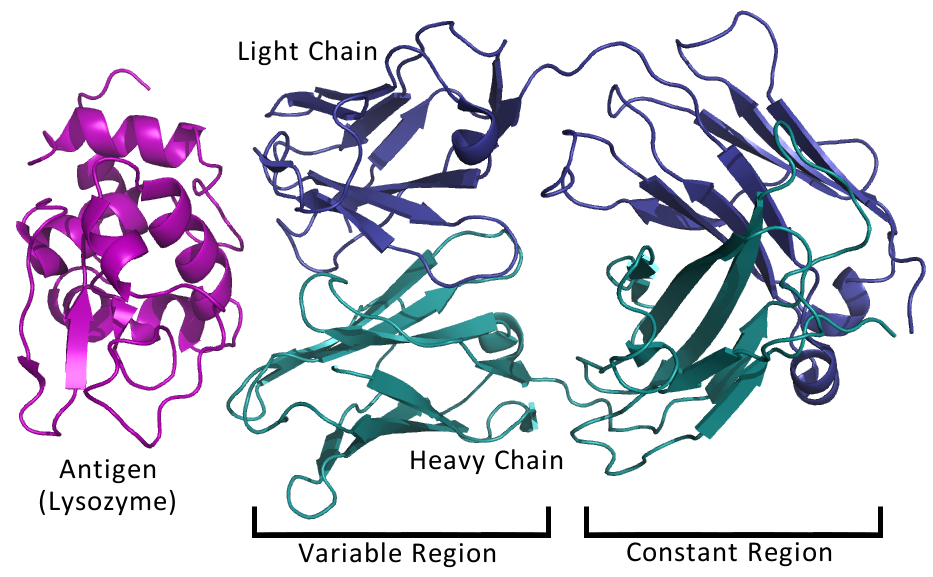
| Molecular Structure of IgG | Antigen Binding within Fab Fragment |
 |
 |
Structural differences between immunoglobulins are used for their classification. As stated above, the type of heavy chain an immunoglobulin possesses determines the immunoglobulin "isotype". More specifically, an isotype is determined by the primary sequence of amino acids in the constant region of the heavy chain, which in turn determines the three-dimensional structure of the molecule. Since immunoglobulins are proteins, they can act as an antigen, eliciting an immune response that generates anti-immunoglobulin antibodies. However, the structural (three-dimensional) features that define isotypes are not immunogenic in an animal of the same species, since they are not seen as "foreign". For example, the five human isotypes, IgA, IgD, IgG, IgE and IgM are found in all humans and a result, injection of human IgG into another human would not generate antibodies directed against the structural features (determinants) that define the IgG isotype. However, injection of human IgG into a rabbit would generate antibodies directed against those same structural features.
Another means of classifying immunoglobulins is defined by the term "allotype". Like isotypes, allotypes are determined by the amino acid sequence and corresponding three-dimensional structure of the constant region of the immunoglobulin molecule. Unlike isotypes, allotypes reflect genetic differences between members of the same species. This means that not all members of the species will possess any particular allotype. Therefore, injection of any specific human allotype into another human could possibly generate antibodies directed against the structural features that define that particular allotypic variation.
A third means of classifying immunoglobulins is defined by the term "idiotype". Unlike isotypes and allotypes, idiotypes are determined by the amino acid sequence and corresponding three-dimensional structure of the variable region of the immunoglobulin molecule. In this regard, idiotypes reflect the antigen binding specificity of any particular antibody molecule. Idiotypes are so unique that an individual person is probably capable of generating antibodies directed against their own idiotypic determinants. This probability forms the basis of the Idiotypic Network Hypothesis to be described later.
Antibodies function in a variety of ways designed to eliminate the antigen that elicited their production. Some of these functions are independent of the particular class (isotype) of immunoglobulin. These functions reflect the antigen binding capacity of the molecule as defined by the variable and hypervariable (idiotypic) regions. For example, an antibody might bind to a toxin and prevent that toxin from entering host cells where its biological effects would be activated. Similarly, a different antibody might bind to the surface of a virus and prevent that virus from entering its host cell. In contrast, other antibody functions are dependent upon the immunoglobulin class (isotype). These functions are contained within the constant regions of the molecule. For example, only IgG and IgM antibodies have the ability to interact with and initiate the complement cascade. Likewise, only IgG molecules can bind to the surface of macrophages via Fc receptors to promote and enhance phagocytosis. The following table summarizes some immunoglobulin properties.
| Isotype | Structure | Placental transfer | Binds mast cell surfaces | Binds phagocytic cell surfaces | Activates complement | Additional features |
|---|---|---|---|---|---|---|
| IgM | 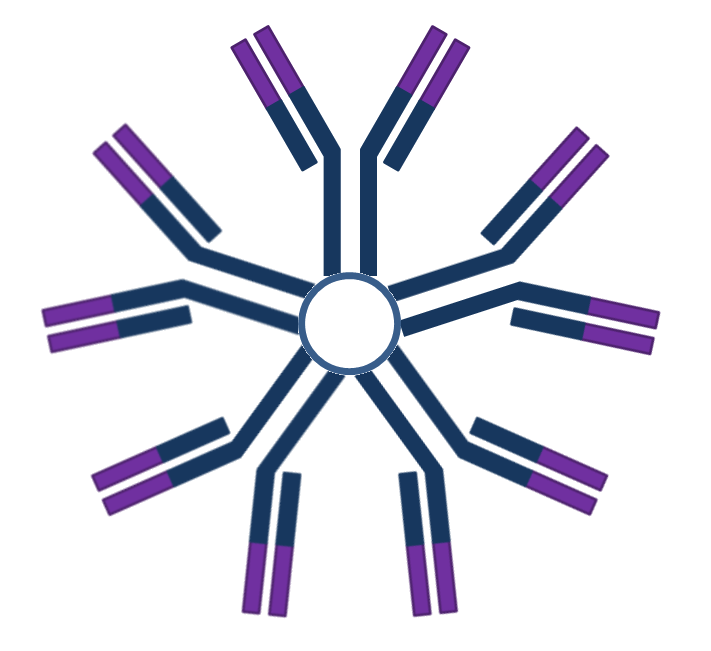 |
- | - | - | + | First Ab in development and response. |
| IgD | 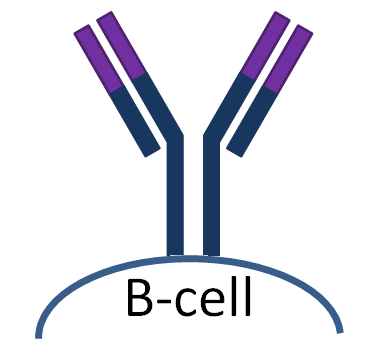 |
- | - | - | - | B-cell receptor. |
| IgG | 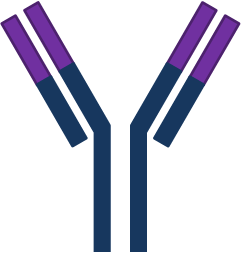 |
+ | - | + | + | Involved in opsonization and ADCC. Four subclasses; IgG1, IgG2, IgG3, IgG4. |
| IgE |  |
- | + | - | - | Involved in allergic responses. |
| IgA | 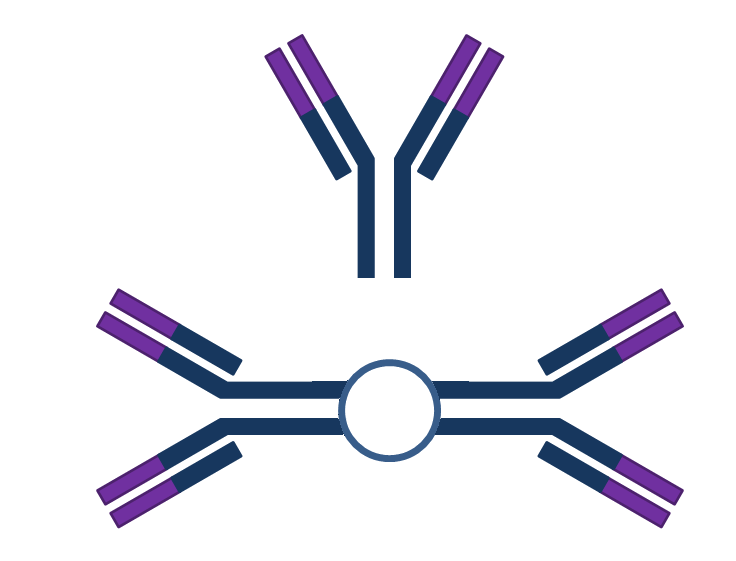 |
- | - | - | - | Two subclasses; IgA1, IgA2. Also found as dimer (sIgA) in secretions. |
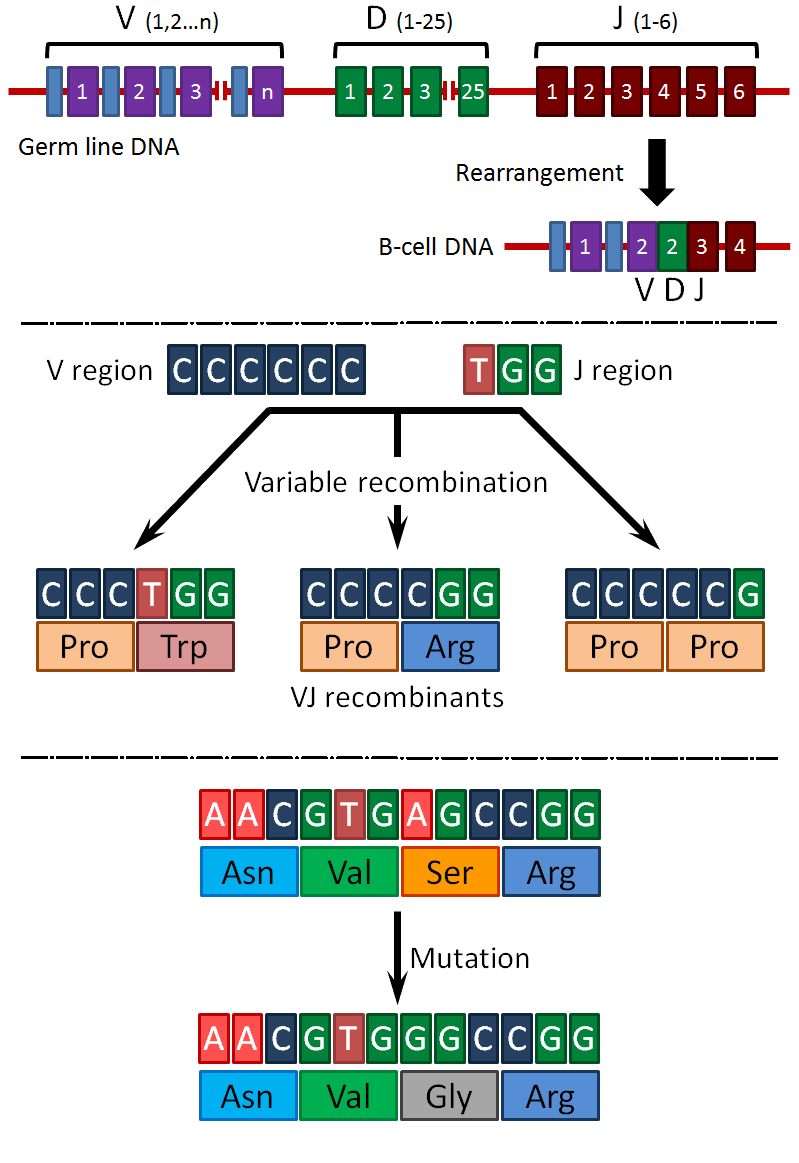
The immune system has the capacity to recognize and respond to about 107 different antigens. This extreme diversity can be generated in at least three possible ways:
It is known that all three of these possibilities take place to produce antibody diversity. The following figures illustrate these possibilities:
The production of immunoglobulins by B-cells or plasma cells occurs in different stages. During differentiation of the B-cells from precursor stem cells, rearrangement, recombination and mutation of the immunoglobulin V, D, and J regions occurs to produce functional VJ (light chain) and VDJ (heavy chain) genes. At this point, the antigen specificity of the mature B-cell has been determined. Each cell can make only one heavy chain and one light chain, although the isotype of the heavy chain may change. Initially, a mature B-cell will produce primarily IgD (and some membrane IgM) that will migrate to the cell surface to act as the antigen receptor. Upon stimulation by antigen, the B-cell will differentiate into a plasma cell expressing large amounts of secreted IgM. Some cells will undergo a "class switch" during which a rearrangement of the DNA will occur, placing the VDJ gene next to the genes encoding the IgG, IgE or IgA constant regions. Upon secondary induction (i.e. the secondary response), these B-cells will differentiate into plasma cells expressing the new isotype. Most commonly, this results in a switch from IgM (primary response) to IgG (secondary response). The factors that lead to production of IgE or IgA instead of IgG are not well understood.