 Left to right: mercury, water and tin cells. You can click on any of the
pictures to enlarge them.
Left to right: mercury, water and tin cells. You can click on any of the
pictures to enlarge them.
 Left to right: mercury, water and tin cells. You can click on any of the
pictures to enlarge them.
Left to right: mercury, water and tin cells. You can click on any of the
pictures to enlarge them.
|
In order to assemble the cell, I melted the tin in the body and assembled the cell while the tin was liquid. Since tin oxidizes readily, I had to find a way to melt the tin in an inert atmosphere. I ended up with a large plastic bucket with a couple of small holes in the bottom to admit a power cord and a hose attached to a nitrogen tank. I put the cell and the furnace in the bucket, put the top loosely on the bucket and started the nitrogen flowing. Once the tin had melted, I reached quickly into the bucket, put the top on the cell and screwed it down tight. It seemed to work pretty well - the tin flowed easily around the cell's stem with none of the oxide crust that I saw when I tried to melt a bit of the tin on the stove.
 Standard Setup
Standard Setup
|
The standard freezing point for pure tin is 231.928 degrees C. At first blush, that sounds pretty hot for a "freezing point" but that's exactly what it is, the temperature where tin freezes from a liquid state to a solid state. As liquid tin cools, the temperature drops to the freezing point then the temperature stays fixed (a "plateau") while the metal changes to a solid state. The same thing happens as it is heated: the temperature rises until the metal starts to change to a liquid. Then the temperature stays constant while the metal changes to a liquid state. Once it is all liquid, the temperature will continue to rise as more heat is applied. One other physical effect complicates the use of a tin cell. Tin can supercool (stay liquid) below the normal freezing point by as much as 10 degrees. Once the solidification starts, however, the cell returns to the freezing point temperature. You can see these effects in the graphs from a test on 4/24/2003:
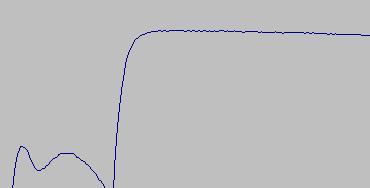
This graph shows the cell freezing. The "bounce" at the start is the supercool. The thermometer started below the temperature of the cell because it had been removed just before the graph was started. See below for more explanation of the procedure.
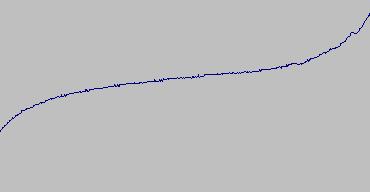
This graph shows the cell melting.
It is clear from the graphs that the freezing plateau is much sharper than the melting plateau. This is due mostly to the level of impurity in the tin. During the gradual freeze, the pure tin tends to freeze at a warmer temperature than the impure tin. This leads to a form of zone purification. When the tin melts, on the other hand, the impure tin melts first and obscures the melt of the pure tin. For these reasons I focused mainly on the freezing plateau.
Here's the general
scheme I am currently using:
Load the cell into the furnace. Put the platinum thermometer in the cell.
Set the temperature controller to 239 degrees (236 + 3).
Heat until the thermometer stops increasing.
Hold at 236 degrees for three hours.
Set the temperature controller to 232 degrees (229 + 3).
Wait for the platinum thermometer to approach 232 degrees.
Remove the top of the furnace and pull the platinum thermometer for 40 seconds.
Put the thermometer and top back on.
Pulling the thermometer cools it to around 200 degrees. When it is re-inserted into the cell, it cools the center of the cell to below the supercool temperature, causing it to begin to begin to freeze. As this graph shows,
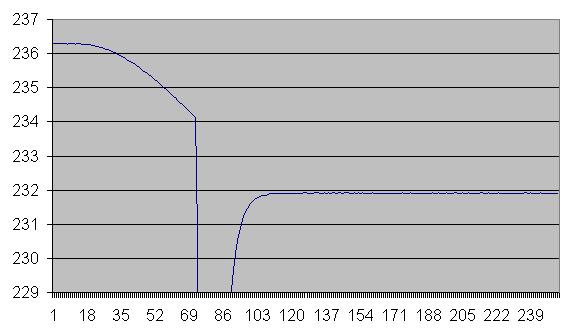
the temperature flattens out nicely. It stays at plus or minus 1/100 degree C for an hour or more. Note that the temperature shown is the result of using the cell to calibrate the thermometer. It is not an independent measurement of the temperature of the cell.