Induction phase: Ag reacts with specific T and B cells
Expansion and Differentiation phase: Induced lymphocyte clones proliferate and mature to a functional stage (i.e. Ag receptor cells mature to Ag effector cells)
Effector phase: Abs or T cells exert biological effects either:
This page will discuss induction, differentiation and regulation of the humoral immune response, focusing on the production of Abs.
Induction of the humoral immune response begins with the recognition of antigen. Through a process of clonal selection, specific B-cells are stimulated to proliferate and differentiate. However, this process requires the intervention of specific T-cells that are themselves stimulated to produce lymphokines that are responsible for activation of the antigen-induced B-cells. In other words, B cells recognize antigen via immunoglobulin receptors on their surface but are unable to proliferate and differentiate unless prompted by the action of T-cell lymphokines. In order for the T-cells to become stimulated to release lymphokines, they must also recognize specific antigen. However, while T-cells recognize antigen via their T-cell receptors, they can only do so in the context of the MHC molecules. This "antigen-presentation" is the responsibility of the antigen-presenting cells (APCs).
Several types of cells may serve the APC function, including the B-cell itself. When B-cells bind antigen, the antigen becomes internalized, processed and expressed on the surface of the B-cell. Expression occurs within the class II MHC molecule, which can then be recognized by T-helper cells (CD4+). Click the image to play or pause the animation.
Other types of antigen-presenting cells include the macrophage and dendritic cells. These cells either actively phagocytose or pinocytose foreign antigens. The antigens are then processed in a manner similar to that observed for the B-cells. Next, specific antigen epitopes are expressed on the macrophage or dendritic cell surface. Again, this expression occurs within the class II MHC molecule, where T-cell recognition occurs. The stimulated T-cells then release lymphokines that act upon "primed" B-cells (B-cells that have already encountered antigen), inducing B-cell proliferation and differentiation. Click the image to play or pause the animation.
B-cells begin their lives in the bone marrow as multipotential stem cells. These completely undifferentiated cells serve as the source for all of the cellular components of the blood and lymphoid system. The initial differentiation step that ultimately leads to the mature B-cell involves DNA rearrangements joining the D and J segments of the immunoglobulin heavy chain genes (click here for more information). Next, DNA rearrangements joining the variable (V) region to the DJ segments of the immunoglobulin heavy chain, as well as similar rearrangements within the light chain genes gives rise to the pre-B-cell. Establishment of the B-cell specificity and consequent expression of surface immunoglobulin gives rise to the "virgin", fully functional B-cell. Each of these steps is entirely independent of antigen.
The antigen-dependent stages of B-lymphocyte differentiation occur in the spleen, lymph nodes and other peripheral tissue. These stages are, of course, initiated upon encounter with antigen and activation by T-cell lymphokines. The activated B-cell first develops into a B-lymphoblast, becoming much larger and shedding all surface immunoglobulin. The B-lymphoblast then develops into a plasma cell, which is, in essence, an antibody factory. This terminal differentiation stage is responsible for production of primarily IgM antibody during the "primary response". Some B-cells, however, do not differentiate into plasma cells. Instead, these cells undergo secondary DNA rearrangements that place the constant region of the IgG, IgA or IgE genes in conjunction with the VDJ genes. This "class switch" establishes the phenotype of these newly differentiated B-cells; these cells remain as long-lived "memory cells". Upon subsequent encounter with antigen, these cells respond very quickly to produce large amounts of IgG, IgA or IgE antibody, generating the "secondary response".
Regulation of the immune response is possibly mediated in several ways. First, a specific group of T-cells, suppressor T-cells, are thought to be involved in turning down the immune response. Like helper T-cells, suppressor T-cells are stimulated by antigen but instead of releasing lymphokines that activate B-cells (and other cells), suppressor T-cells release factors that suppress the B-cell response. While immunosuppression is not completely understood, it appears to be more complicated than the activation pathway, possibly involving additional cells in the overall pathway.
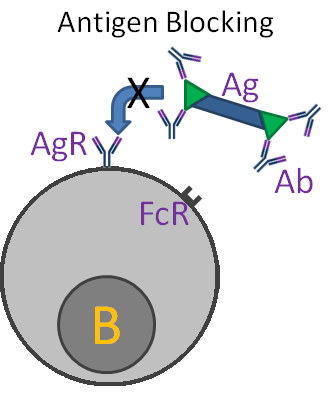
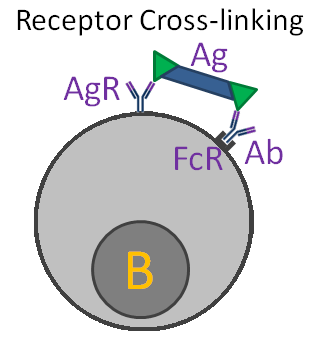
Other means of regulation involve interactions between antibody and B-cells. One mechanism, "antigen blocking", occurs when high doses of antibody interact with all of the antigen's epitopes, thereby inhibiting interactions with B-cell receptors. A second mechanism, "receptor cross linking", results when antibody, bound to a B-cell via its Fc receptor, and the B-cell receptor both combine with antigen. This "cross-linking" inhibits the B-cell from producing further antibody.
Another means of regulation that has been proposed is the idiotypic network hypothesis. This theory suggests that the idiotypic determinants of antibody molecules are so unique that they appear foreign to the immune system and are, therefore, antigenic. Thus, production of antibody in response to antigen leads to the production of anti-antibody in response, and anti-anti-antibody and so on. Eventually, however, the level of [anti]n-antibody is not sufficient to induce another round and the cascade ends. Click the image to play or pause the animation.