
The Major Histocompatibility Complex (MHC) is a set of molecules displayed on cell surfaces that are responsible for lymphocyte recognition and "antigen presentation". The MHC molecules control the immune response through recognition of "self" and "non-self" and, consequently, serve as targets in transplantation rejection. The Class I and Class II MHC molecules belong to a group of molecules known as the Immunoglobulin Supergene Family, which includes immunoglobulins, T-cell receptors, CD4, CD8, and others. This page will describe the MHC molecules and the process of antigen presentation.

The major histocompatibility complex is encoded by several genes located on human chromosome 6. Class I molecules are encoded by the BCA region while class II molecules are encoded by the D region. A region between these two on chromosome 6 encodes class III molecules, including some complement components.
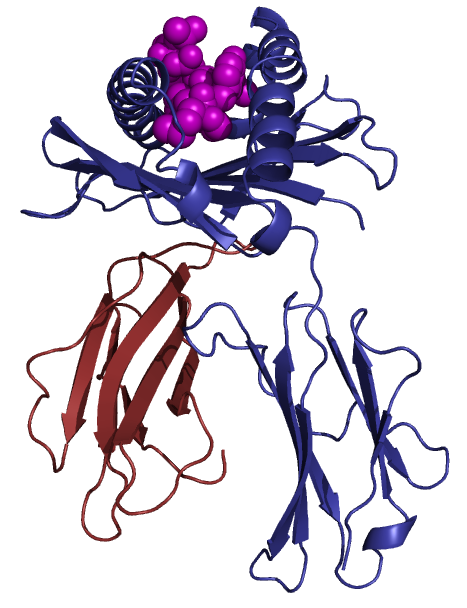
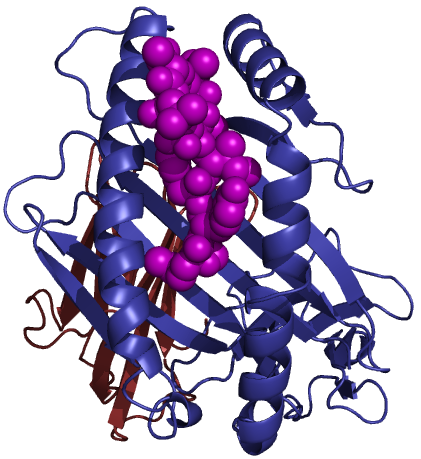
Class I molecules are composed of two polypeptide chains; one encoded by the BCA region and another (Β2-microglobulin) that is encoded elsewhere. The MHC-encoded polypeptide is about 350 amino acids long and glycosylated, giving a total molecular weight of about 45 kDa. This polypeptide folds into three separate domains called alpha-1, alpha-2 and alpha-3. Β2-microglobulin is a 12 kDa polypeptide that is non-covalently associated with the alpha-3 domain. Between the alpha-1 and alpha-2 domains lies a region bounded by a beta-pleated sheet on the bottom and two alpha helices on the sides. This region is capable of binding (via non-covalent interactions) a small peptide of about 10 amino acids. This small peptide is "presented" to a T-cell and defines the antigen "epitope" that the T-cell recognizes (see below). The following images illustrate the structure of the class I MHC as seen schematically, and three dimensionally from the side and from the top (T-cell perspective). The MHC-encoded polypeptide is shown in blue, the Β2-microglobulin is green and the peptide antigen is red.
| Class I MHC | Side view | Top view |
|---|---|---|
|
Binding 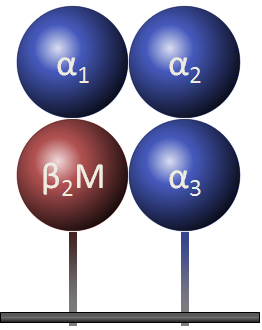
|
 |
 |
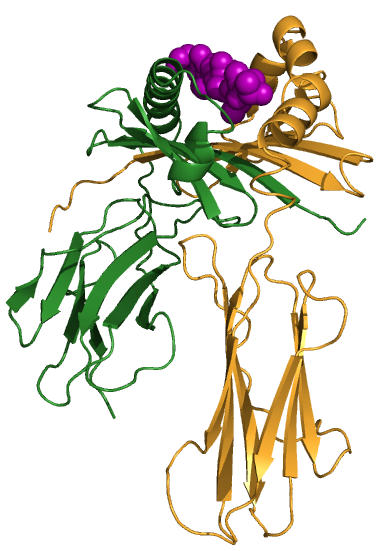
Class II molecules are composed of two polypeptide chains, both encoded by the D region. These polypeptides (alpha and beta) are about 230 and 240 amino acids long, respectively, and are glycosylated, giving molecular weights of about 33 kDa and 28 kDa. These polypeptides fold into two separate domains; alpha-1 and alpha-2 for the alpha polypeptide, and beta-1 and beta-2 for the beta polypeptide. Between the alpha-1 and beta-1 domains lies a region very similar to that seen on the class I molecule. This region, bounded by a beta-pleated sheet on the bottom and two alpha helices on the sides, is capable of binding (via non-covalent interactions) a small peptide of about 10 amino acids. This small peptide is "presented" to a T-cell and defines the antigen "epitope" that the T-cell recognizes (see below). The following images illustrate the structure of the class II MHC as seen schematically, and three dimensionally from the side and from the top (T-cell perspective). The MHC-encoded polypeptides are shown in yellow and green, while the peptide antigen is shown in red.
| Class II MHC | Side view | Top view |
|---|---|---|
|
Binding 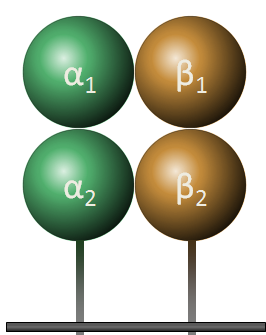
|
 |
 |
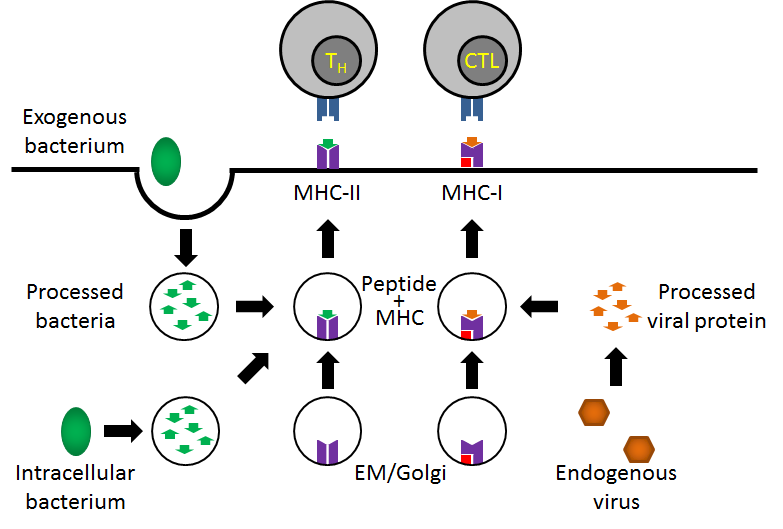
While class I and class II molecules appear somewhat structurally similar and both present antigen to T-cells, their functions are really quite distinct. First, class I molecules are found on virtually every cell in the human body. Class II molecules, in contrast, are only found on B-cells, macrophages and other "antigen-presenting cells" (APCs). Second, class I molecules present antigen to cytotoxic T-cells (CTLs) while class II molecules present antigen to helper T-cells (TH-cells). This specificity reflects the third difference, the type of antigen presented. Class I molecules present "endogenous" antigen while class II molecules present "exogenous" antigens. An endogenous antigen might be fragments of viral proteins or tumor proteins. Presentation of such antigens would indicate internal cellular alterations that if not contained could spread throughout the body. Hence, destruction of these cells by CTLs is advantageous to the body as a whole. Exogenous antigens, in contrast, might be fragments of bacterial cells or viruses that are engulfed and processed by e.g. a macrophage and then presented to helper T-cells. The TH-cells, in turn, could activate B-cells to produce antibody that would lead to the destruction of the pathogen.
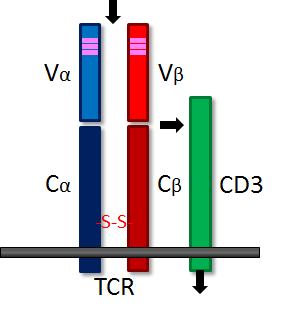
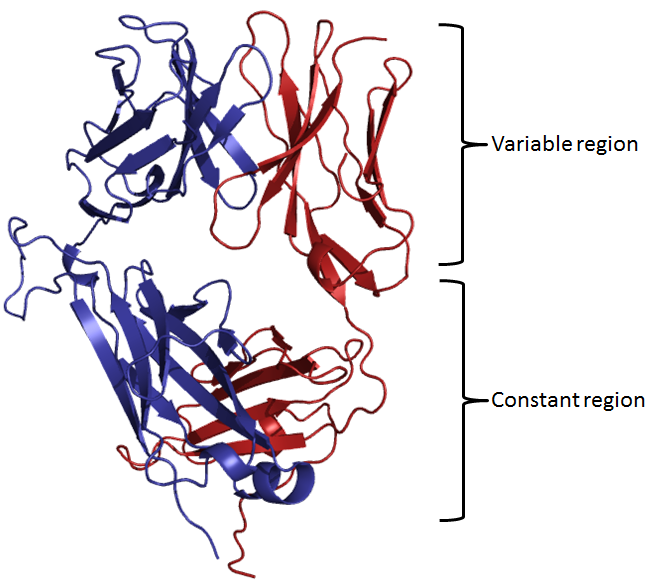
The T-cell receptor molecule (TCR) is structurally and functionally similar to the B-cell
immunoglobulin receptor. TCR is composed of two, disulfide-linked polypeptide chains, alpha and beta, each having separate constant and variable domains much like immunoglobulins. The variable domain contains three hypervariable regions that are responsible for antigen recognition. Genetic diversity is ensured in a manner analogous to that for immunoglobulins (click here for more information). Thus, just like the B-cell surface immunoglobulin provides antigen specificity to its B-cell, the TCR allows T-cells to recognize their particular antigenic moiety. However, T-cells cannot recognize antigen without help; the antigenic determinant must be presented by an appropriate (i.e. self) MHC molecule. Upon recognition of a specific antigen, the signal is passed to the CD3 molecule and then into the T-cell, prompting T-cell activation and the release of lymphokines. The following images illustrate the structure of the TCR as seen schematically, and three dimensionally from the side.
| TCR | Side view |
|---|---|
|
 |
The TCR provides the specificity for an individual T-cell to recognize its particular antigen. However, this recognition is "MHC-restricted" because the TCR also requires interactions with MHC. Also, interactions between the CD4 molecule (found on helper T-cells) and class II MHC or the CD8 molecule (found on cytotoxic T-cells) and class I MHC stabilize and consummate the antigen recognition process, allowing helper T-cells to respond to "exogenous" antigens (leading to B-cell activation and the production of antibody) or cytotoxic T-cells to respond to "endogenous" antigens (leading to target cell destruction). The following images illustrate these processes schematically, and three dimensionally.
| TCR - APC (class II) | TCR - Target cell (class I) | |
|---|---|---|
 |
 |
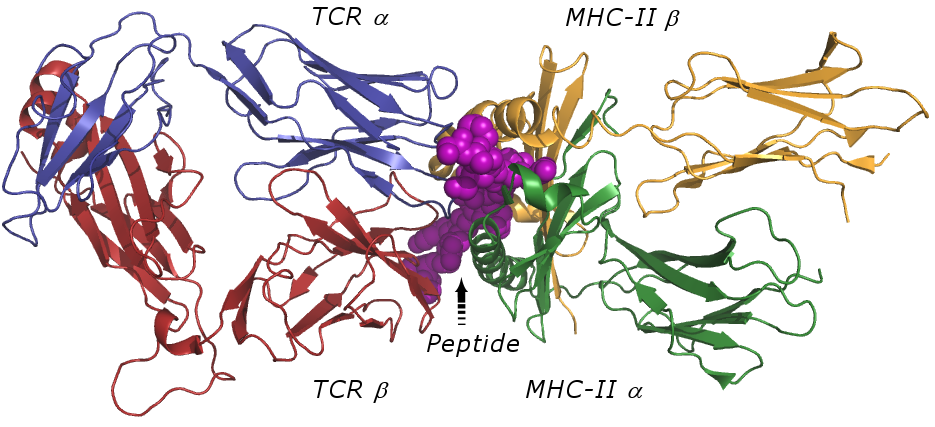
| Antigen Presentation by MHC-II to TCR |
|---|
 |